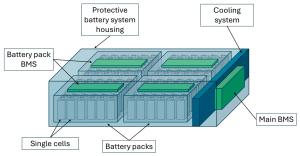
Why Lithium Ion Phosphate Batteries?
Lithium Ion Phosphate batteries, often known as LFP batteries or LiFePO4 batteries due to their chemical composition, will be the focus of this article. These batteries differ slightly from the lithium-cobalt batteries that you typically see in laptops and cell phones. LFP has the advantage of being significantly more stable and resistant to self-combustion. A charged battery has a ton of energy stored in it, so in the event of an unintentional discharge, the outcomes might be very interesting. However, this does not mean the battery cannot catch fire in the event of damage. In addition to lasting longer than lithium-cobalt, LFP is more temperature stable.

Why use lithium ion?
In our article on lead-acid batteries, we discussed how this chemistry’s Achilles heel is resting at partial charge for an extended period of time. By leaving a costly lead-acid battery bank at the partial charge, it is far too simple to squander it in a matter of months. For LFP, that is extremely different! Lithium-ion batteries are unaffected by prolonged partial charging. In fact, LFP prefers partial charge to full or empty charge, so it is advisable to cycle the battery or leave it at partial charge for its lifespan.
With the proper charge settings, you could almost forget there is a battery inside, which makes lithium-ion batteries incredibly close to becoming the ideal battery. There isn’t any upkeep. You may cycle away with ease knowing that the BMS will take care of it!
Additionally, LFP batteries have a very long lifespan. At a complete 100% charge/discharge cycle, our Battle Born LFP batteries are rated for 3000 cycles. That would amount to more than 8 years of riding if you did it every day! When used in less-than-100% cycles, they survive even longer; in fact, for simplicity, a linear connection can be employed: 50% discharge cycles translates into twice as many cycles, whereas 33% discharge cycles indicate that you can expect three times as many cycles.
Additionally, a LiFePO4 battery weighs less than half as much as a lead-acid battery of equivalent capacity. Try charging a lead-acid battery at 100% of its rated capacity; it can handle it with ease. It is sealed to prevent odors, has a very low self-discharge rate (3% or less per month), and supports rapid charging.

Size of the Battery Bank for LFP
We alluded to this earlier: Lead-acid batteries only have an effective capacity of 80%, while lithium-ion batteries have 100%. This means that an LFP battery bank can be smaller in size than a lead-acid battery bank without losing any functionality. The calculations indicate that LFP may be 80% the size of lead acid in amp-hours. But there’s more to it than that.
Lead-acid battery banks shouldn’t be sized so that they frequently experience draining below 50% SOC for the sake of longevity. That is not a problem with LFP! LFP also has a far higher round-trip energy efficiency than lead-acid batteries, which means that after a certain amount of depletion, less energy is required to refuel the tank. Due to the fact that our battery bank was previously lower, this causes a quicker return to 100%, amplifying the effect.
We are confident that when developing a lithium-ion battery bank between 55% and 70% of the size of an identical lead-acid bank, the performance will be the same (or better!). Even on those gloomy winter days when the sun is scarce.
But hold on a second! Are lithium-ion batteries truly the answer to all of our battery woes? Okay, not quite...
LFP batteries have restrictions as well. Temperature is a major one. Below 0 degrees Celsius, or freezing, a lithium-ion battery cannot be charged. Lead acid is unconcerned with this. The battery can still be discharged (with a brief loss of capacity), but charging will not occur. The BMS should be cautious to prevent charging when it is below zero degrees to prevent unintentional damage. In the climate we have in Canada, this is a Big Deal!
The top end also has a problem with temperature. Use or even merely storage of batteries at high temperatures is the single biggest factor in battery aging. There are no issues in temperatures below 30 Celsius. There aren’t many effects at temperatures lower than 45 degrees. But anything greater truly hastens to age and, eventually, the battery’s demise. The battery must be stored when it isn’t being cycled. When we explain how LFP batteries fail later, we will go into more detail about this.

There is a hidden issue that might occur when using charging sources that have the potential to give a high Voltage: If the charging source doesn’t stop when the battery is full, the Voltage will rise. When the battery rises high enough, the BMS will disconnect it to protect it, allowing the charging source to rise even further. This may be a problem with (faulty) automobile alternator voltage regulators, which require a constant load to prevent voltage spikes and the magical smoke from diodes. This issue can also arise with small wind turbines that rely on the battery to keep them in check. When the power runs out, they can flee. The initial purchasing cost is also extremely high.







-300x300.jpg)


