Lithium-ion batteries power the lives of innumerable individuals every day. From laptops and cell phones to hybrids and electrical cars, this technology is growing in quality thanks to its lightweight weight, high energy density, and talent to recharge. Do you know how does 12v lithium-ion battery work? Let’s move, this passage can tell you.
How Does lithium ion battery Charge and Discharge
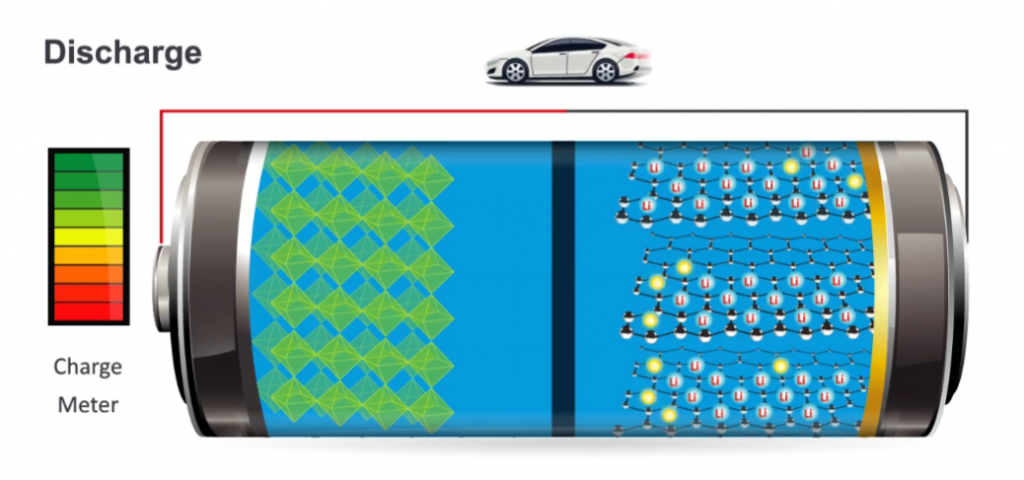
As its name suggests, lithium ion battery 18650 square measure all concerning the movement of lithium ions: the ions move one way once the battery charges (when it is absorbing power); they move the other way once the battery discharges (when it is supplying power).
Charge:
During charging, lithium ions (yellow circles) result in the positive conductor (red) to the negative conductor (blue) through the solution (gray). Electrons conjointly result in the positive conductor to the negative conductor, however, take the longer path around the outer circuit. The electrons and ions mix at the negative conductor and deposit lithium ions there.
When no additional ions can flow, the lithium-ion battery 12v is absolutely charged and prepared to use.
Discharge:
During discharging, the ions flow back through the solution from the negative conductor to the positive conductor. Electrons result in the negative conductor to the positive conductor through the outer circuit, powering your laptop computer. Once the ions and electrons mix at the positive conductor, lithium-ion is deposited there. When all the ions have captive back, the battery is absolutely discharged and wishes to charge up once more.
How Is Lithium-ion Stored

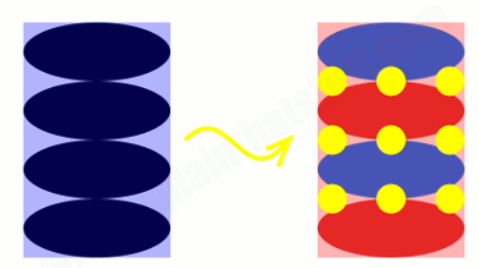
The diagram is showing however lithium ion particles migrate back and forth in exceedingly 12v lithium ion batteries. This second animation shows what is going on within the battery in an exceedingly bit additional detail. Again, the negative graphite electrode (blue) is shown on the left, the positive cobalt-oxide conductor (red) on the proper, and also the lithium ion ions square measure delineated by yellow circles. Once the battery is absolutely charged, all the lithium ions square measure keep between layers of graphene (sheets of carbon one atom thick) within the graphite electrode (they have all captive over to the left).
During this charged-up state, the battery is effectively a multi-layer sandwich: graphene layers alternate with lithium ion particle layers. Because the battery discharges, the ions migrate from the graphite electrode to the cobalt-oxide conductor (from left to right). Once it’s absolutely discharged, all the lithium ions have captive over to the cobalt-oxide conductor on the proper. Once again, the lithium ions sit in layers, in between layers of lithium ions (red) and chemical compound ions (blue). Because the battery charges and discharges, the lithium ions shunt back and forth from one conductor to the opposite.
How Does 12v lithium ion battery Work
The basics
A battery is formed from an associate anode, cathode, separator, solution, and 2 current collectors (positive and negative). The anode and cathode store the lithium ion. The solution carries charged lithium ions from the anode to the cathode and contrariwise through the centrifuge. The movement of the lithium ions creates free electrons within the anode that creates a charge at the positive current collector. The electrical current then flows from this collector through a tool being supercharged (cell phone, computer, etc.) to the negative current collector. The centrifuge blocks the flow of electrons within the battery.
Charge and discharge
While the battery is discharging and providing an electrical current, the anode releases lithium ions to the cathode, generating a flow of electrons from one aspect to the opposite. Once plugged within the device, the other happens: lithium ions square measure discharged by the cathode and received by the anode.
Energy density VS. Power density
The two most typical ideas related to batteries square measure energy density and power density. Energy density is measured in watt-hours per kilo (Wh/kg) and is the quantity of energy the battery will store with relevance to its mass. Power density is measured in watts per kilo (W/kg) and is the quantity of power that will be generated by the battery with relevancy its mass. To draw a clearer image, consider exhausting a pool. Energy density is comparable to draining the pool as quickly as possible. whereas power density is resemble exhausting the pool as quickly as attainable. We have a tendency to work on increasing the energy density of batteries, whereas reducing the value, and maintaining a suitable power density.
Do lithium batteries leak acid?
Lithium batteries generally do not leak. When you buy a device, check the battery required to be sure you are using the right battery or that you don’t mistake an alkaline battery for a lithium battery.
Conclusion:
Lithium-ion batteries square measure unbelievably fashionable recently. You’ll be able to realize them in. they are therefore common as a result of, pound for pound, and they are a number of the foremost energetic reversible batteries obtainable. If you want to know more about lithium ion battery prices, please contact lithium-ion battery manufacturer Maxworld Power.

